Method Validation in HPLC
What is Method Validation in HPLC?
Method validation is a process of verifying an analytical method used in hplc to test the sample. This step ensures to produces reliable, consistent, and accurate results under defined conditions.
Importance Method Validation
Method validation in HPLC is a plays critical step to ensure data integrity and regulatory compliance. Regulatory bodies require validated methods for any process with drug development, manufacturing, or release. Without method validation this results may be unreliable.
Key Parameters of Method Validation
- Accuracy-To Measures how close the test result is to true value.
- Precision– To Evaluate the reproducibility of method under normal operating condition.
- Specificity– To determine the method accuracy to measure the analyte in presence of other components like impurities, excipients, or degradation products.
- Linearity– To check if the test results are directly proportional to the analyte concentration range Limit of Detection (LOD)-lowest amount of analyte that can be detected
- Limit of Quantification (LOQ)-lowest amount of analyte that can be quantitatively measured.
- Robustness-To check if small, deliberate changes in method parameters (like flow rate, temperature, pH) affect the results.
- System Suitability Testing-To check if HPLC system is functioning properly
Accuracy
What is Accuracy in HPLC Method Validation?
Accuracy refers to the closeness of true value to the value found using the HPLC method. To ensure that the method quantifies the analyte without overestimating or underestimating the actual concentration.
Importance of Accuracy
To Ensuring that reported results reflect the true concentration
To Meet regulatory requirements
To Verifying product quality especially in pharmaceuticals
How is Accuracy Evaluated ?
- Spike the known amount of the analyte into the sample matrix (like placebo or excipients).
- Run this multiple concentration spiked sample covering the full range in the method (e.g., 50%, 100%, and 150% of the target concentration).
- Calculating the percent recovery of the spiked analyte.
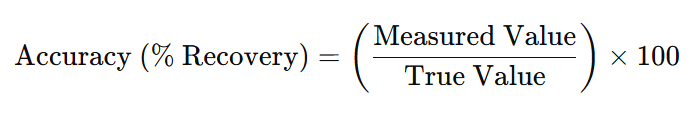
For assay of drug substance or finished product acceptance range of 98% to 102% is acceptable
For impurity a wider range of 80% to 120% is acceptable.
Specificity
What is Specificity in HPLC Method Validation?
Specificity in HPLC method validation refers to the method’s ability to clearly produce the peak for the analyte of interest in the presence of potential interference peaks ( impurities, degradation products, matrix components, or other active ingredients).
Importance of Specificity
- To ensure accuracy and reliability of test results.
- To help distinguish analytes from similar compounds or matrix noise.
- To separate degradateable products from the main compound.
How is Specificity Evaluated
- Inject blank, placebo, standard, and sample solutions.
- Compare chromatograms for any co-eluting peaks overlaps.
- Analyte peak should be well-resolved from all other peaks
- While specificity is mostly qualitative, you can support it using peak purity analysis or resolution (Rs) between peaks.
Linearity
What is Linearity in HPLC Method Validation?
Linearity in HPLC method validation verifies the ability of a method to produce results that are directly proportional to the range concentration and accurately measure different concentrations of the analyte.
Importance of Linearity
- To ensure the method can accurately quantify the analyte of various concentration levels.
- Without this step the method may be unreliable at low or high concentrations even if it works well in the middle of the range.
- To confirm the suitability of the method for assay, impurity testing or stability studies.
How is Linearity Evaluated ?
- Prepare at least 5 different concentrations of the analyte covering 80% to 120% of target concentration .
- Inject each concentration in the HPLC system.
- Record and Plot a calibration curve:
Perform linear regression analysis to determine Slope,Intercept and Correlation coefficient (R²)
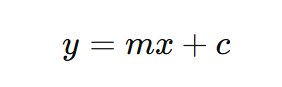
Where:
- y = detector response (peak area)
- x = analyte concentration
- m = slope
- c = intercept
Linearity is usually confirmed by:
- A correlation coefficient (R²) ≥ 0.999 for assay methods
- Consistent residuals across the curve
- Visual inspection of the plotted curve
Precision
What is Precision in HPLC Method Validation?
Precision in HPLC method validation refers to closeness of agreed value between a series of measurement values from multiple samples of the same homogeneous sample, under the same conditions.
This is evaluated in two parts:
- System precision in HPLC
- Repeatability in HPLC (also called method precision)
What is System Precision in HPLC?
System precision in HPLC to find the consistency of the HPLC instrument, specifically how well the instrument performs on repeated injections of the same standard solution. This process reflects the performance of the HPLC system, including injector, detector, pump, and column.
Performed by making 5–6 replicate injections of a standard solution and finding %RSD (Relative Standard Deviation) of peak area or retention time.
What is Repeatability in HPLC (Method Precision)?
Repeatability or intra-day precision in HPLC is done to find the variability of results when the entire analytical procedure is repeated under the same conditions. Done to assesses the analyst’s technique by analyzing multiple preparations from the same batch
Importance of Precision
- To Ensure the reliability and reproducibility of results over time.
- Verify method is stable and controlled to produce a valid result.
- Required by regulatory agencies to confirm method performance.
Precision Calculate using the Relative Standard Deviation (RSD) using formula.

LOD (Limit of Detection) / LOQ (Limit of Quantification)
What is LOD?
LOD (Limit of Detection) in HPLC method validation is done to find the lowest amount of analyte that can be detected but not necessarily quantified under the stated method. This is used to indicate the method’s sensitivity and analyte peak must be visible and understandable from baseline noise
What is LOQ?
LOQ (Limit of Quantification) in HPLC method validation is done to find the lowest amount of analyte that can be quantified. This value is always higher than LOD for the same method.
Importance of LOD and LOQ
- Critical for impurity profiling,stability studies and trace analysis
- Required by regulatory agencies and also Help establish the working range in HPLC method validation.
- To ensure sensitive detection, especially in pharmaceuticals.
How to find and calculate LOD and LOQ in HPLC (Signal-to-Noise Method)
- Inject a dilute solution of the analyte
- Measure the peak height (signal) and baseline noise near the peak region.
- Calculate the S/N ratio by using software or manually as
LOD=Concentration at which S/N ratio is approximately 3:1( which means that the analyte signal is three times higher than background noise)
LOQ=Concentration at which S/N ratio is approximately 10:1(which means that the analyte signal is ten times higher than the noise)
