Testing Procedure L4: Multi-drug Tablet (Compound E and Compound F) Dosage Form
Determination of Compound E and Compound F Purity in Tablet Dosage Form Using RP-HPLC
(Using 20 Tablets, Each Containing 100 mg Compound E and 150 mg Compound F )
Important Disclaimer :The following procedure is intended solely for learning and demonstration purposes. It is not validated or approved for actual laboratory use. This procedure should never be used in any real analytical or pharmaceutical setting. Values, volumes, and methods are simplified to help learners understand the basic workflow involved in HPLC sample preparation.
1. Objective
To quantitatively determine the content of Compound E and Compound F in combination tablets using reverse-phase high-performance liquid chromatography (RP-HPLC), via a single injection providing two distinct peaks.
2. Materials and Equipment
Chemicals
- Compound E reference standard (purity ≥ 99.8%)
- Compound F reference standard (purity ≥ 99.8%)
- Fixed-dose combination tablets (Each tablet contains 100 mg of Compound E and 150 mg of Compound F)
- HPLC-grade acetonitrile
- Potassium dihydrogen phosphate (KH₂PO₄)
- 1 M potassium hydroxide (KOH)
- Distilled water
Equipment
HPLC system with UV detector (254 nm)
C18 HPLC column (150 mm × 4.6 mm, 3.5 µm)
pH meter
Analytical balance
0.45 µm membrane filters
Volumetric flasks (10 mL, 100 mL, 200 mL)
Ultrasonicator
Centrifuge
Syringes
3. Methodology
3.1Mobile Phase Preparation Procedure
3.1.1 Phosphate Buffer (pH 6.5):
Weighing and Dissolving:
Accurately weigh 6.8 grams of potassium dihydrogen phosphate (KH₂PO₄).
Transfer the salt into a beaker containing approximately 800 mL of distilled water.
Stir the solution using a magnetic stirrer or glass rod until the KH₂PO₄ is completely dissolved.
pH Adjustment:
Using a calibrated pH meter, monitor the pH of the solution.
Adjust the pH to 6.5 by adding 1 M potassium hydroxide (KOH) solution dropwise while stirring continuously.
After reaching the desired pH, transfer the solution to a 1000 mL volumetric flask and make up the volume to the mark with distilled water.
3.1.2 Final Mobile Phase:
Mobile Phase A: Phosphate Buffer (pH 6.5)
Prepare the phosphate buffer as described, ensuring the pH is adjusted to 6.5.
Transfer the prepared buffer into a clean, labeled container for use as Mobile Phase A.
Mobile Phase B: Acetonitrile (HPLC Grade)
Use HPLC-grade acetonitrile as the organic phase for the gradient.
Ensure the acetonitrile is properly stored in a clean, tightly sealed container to prevent contamination.
Filtration and Degassing:
Filter both Mobile Phases (A and B) through 0.45 µm filters to remove particulate matter.
Degas both phases using ultrasonication for 10–15 minutes or by applying vacuum, ensuring the elimination of dissolved gases that could affect the chromatographic results.
3.2 Preparation of Dissolving solvent
Mix 20 mL distilled water with 80 mL acetonitrile (20:80 v/v)
3.3 Preparation of Solutions
3.3.1 Standard Solution
- Accurately weigh 10.0 mg of Compound E and 15.0 mg of Compound F
- Transfer both to the same 100 mL volumetric flask
- Dissolve in 20:80 water:acetonitrile(Dissolving solvent)
- Sonicate if necessary
- This is a 100 μg/mL Compound E + 150 μg/mL Compound F stock
- Pipette 1.0 mL of stock into a 10 mL volumetric flask
- Dilute with Dissolving solvent
- Final concentration: 10 μg/mL Compound E, 15 μg/mL Compound F,
- Filter using a 0.45 μm syringe filter before HPLC analysis
3.3.2 Sample Solution (10 μg/mL)
Step 1: Preparation of Stock Solution
Collect and accurately count 20 tablets containing Compound E and Compound F.
Weigh the 20 tablets .
Pulverize the tablets into a fine, uniform powder using a clean mortar and pestle.
Weigh 0.1 g of the powdered tablet mixture, equivalent to 10 mg of Compound E and 15 mg of Compound F.
Transfer the 0.1 g powder into a 100 mL volumetric flask.
Add approximately 70 mL of Dissolving solvent to the flask.
Sonicate the mixture for 15–20 minutes to ensure complete extraction of Compounds.
Allow the solution to cool to room temperature.
- Centrifuge at 4000–5000 rpm for 10 minutes (if needed)
- Collect the supernatant
- Make up to volume with the same dilution solvent
Step 2: Preparation of Working Solution
Pipette 1.0 mL of the stock solution into a 10 mL volumetric flask.
Dilute to the mark with Dissolving solvent to obtain a working solution of 10 μg/mL of Compound E and 15 μg/mL of Compound F.
Filter the working solution through a 0.45 μm syringe filter to remove particulate matter.
3.4.1 HPLC Conditions
| Parameter | Value |
|---|---|
| Column | C18, 150 mm × 4.6 mm, 3.5 μm |
| Mobile Phase | Gradient Program |
| Flow Rate | 1.2 mL/min |
| Injection Volume | 20 μL |
| Detection Wavelength | 254 nm |
| Column Temperature | 30°C |
| Run Time | ~20 minutes |
| Retention Times | Compound E: ~3 minutes (expected) Compound F: ~6 minutes (expected) |
3.4.2 HPLC Gradient Program
| Time (min) | Mobile Phase A (%)(Buffer) | Mobile Phase B (%)(Acetonitrile) |
|---|---|---|
| 0 – 2 | 80% | 20% |
| 2 – 5 | 70% | 30% |
| 5 – 8 | 50% | 50% |
| 8 – 10 | 20% | 80% |
| 10 – 12 | 80% | 20% |
4. Sample Analysis
Inject the standard and sample solutions under the specified HPLC conditions.
Record the retention times and peak areas.
Confirm identity by comparing sample retention time with that of the standard.
Calculate the % purity of Compound E and Compound F in the tablet.
5. Calculation of Purity
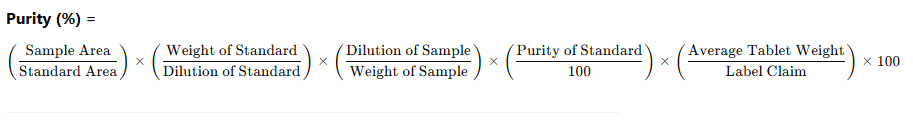
Weight of standard used: 10.0 mg
Weight of sample (API): 100.0 mg (from 0.100 g powder)
Acceptance Criteria: 98.5% – 101.5%
6. Reporting
Report must include:
Tablet strength and total weight used (20.000 g)
Sample preparation steps
Chromatograms with retention times and peak areas
Calculation details and % purity result
Analyst and reviewer signatures
Observations or deviations
7. Safety and Waste Disposal
Wear appropriate PPE (gloves, goggles, lab coat)
Handle Acetonitrile in a fume hood (flammable and toxic)
- Re-equilibration: Maintain 80% A / 20% B for 5 minutes after run to allow column reconditioning.
Dispose of solvents and samples according to local safety and environmental regulations
You’ve just reviewed the preparation steps used in HPLC analysis. Now it’s time to apply your knowledge.
Click the button below to proceed
Important Disclaimer:The following procedure is intended solely for learning and demonstration purposes. It is not validated or approved for actual laboratory use. This procedure should never be used in any real analytical or pharmaceutical setting. Values, volumes, and methods are simplified to help learners understand the basic workflow involved in HPLC sample preparation.